Immune Checkpoint Inhibitor Colitis: A 15-Point Management Protocol
Decision-Making Framework for Diagnosis, Risk Stratification, and Therapeutic Intervention in ICI-Associated Gastrointestinal Toxicity
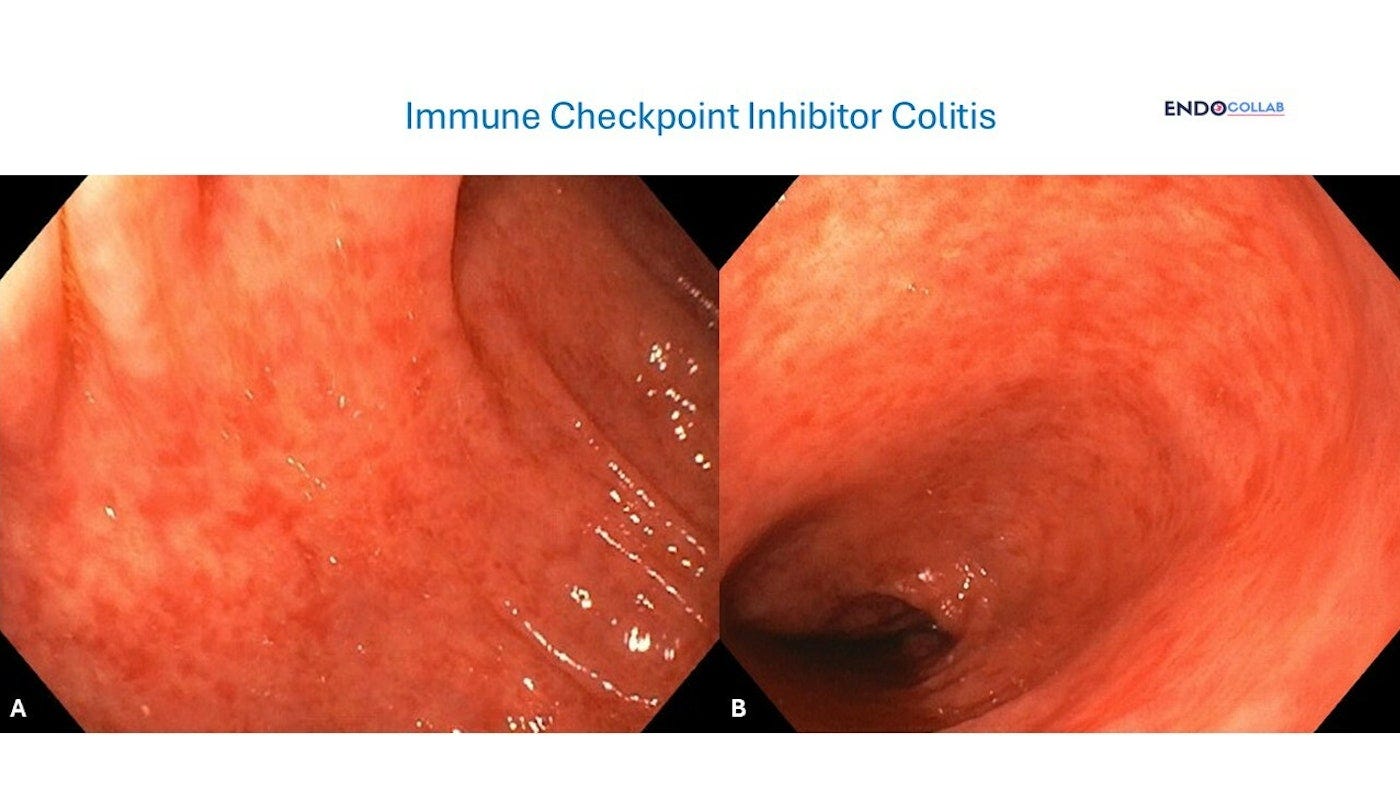
Patient with melanoma on ipilimumab and nivolimumab.
AGA Guidelines: AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review - ClinicalKey
Best practice advice
1. Infectious causes of diarrhea should be excluded before treatment of suspected immune checkpoint inhibitors (ICI) colitis.
2. Early stool testing for inflammatory markers (lactoferrin and calprotectin) in patients with grade 2 or higher colitis/diarrhea (more than four bowel movements daily above baseline) according to the Common Terminology Criteria for Adverse Events, Version 5 (CTCAE) and selected patients with less-severe diarrhea may help stratify high-risk patients for endoscopic evaluation.
3. Endoscopic confirmation of the diagnosis and severity of ICI colitis should be considered before initiation of high-dose systemic glucocorticoids.
4. Abdominal imaging may be considered to exclude serious complications in patients with dominant symptoms of pain, fever, or bleeding, but should not be performed routinely in patients with diarrhea alone.
5. Rapid progression of ICI colitis may occur within a period of days, particularly in patients treated with ipilimumab and, therefore, prompt diagnosis and treatment is required.
6. ICI colitis typically responds to high-dose systemic glucocorticoids, given in doses of 0.5-2 mg/kg prednisone equivalent daily with a taper of 4-6 weeks; however, these doses and schedules have not been rigorously examined. Infliximab and vedolizumab are reasonable options for treatment of glucocorticoid refractory colitis.