In this video-based article, Prof. Klaus Mönkemüller, MD, PhD, presents a concise overview of the development and clinical application of hemostatic powders, detailing the mechanism, use cases, and limitations of three distinct generations of products.
Imagine you’re scoping a patient with a posterior duodenal ulcer. It’s spurting, and the angle is difficult. You apply clips, but they slide off. You inject, but the bleeding continues. This is a classic scenario where endoscopists are now turning to hemostatic powders, not as a final therapy, but as a critical temporizing measure.
This is the concept of “down-staging”—one of several key applications for these agents. We’ll explore this, along with rescue therapy and the critical differences in how these powders are made and, most importantly, how they are applied.
Part 1: The First Generation – Hemospray (TC 325)
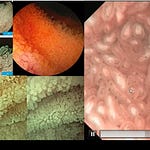
The first-generation agent, Hemospray (TC 325), is an inorganic mineral powder.
The Mechanism: “It’s Clay”
The speaker simplifies the product’s composition: “it’s clay”. Specifically, it is a bentonite powder, a type of kaolin, which is a naturally occurring aluminum phyllosilicate mineral.
Its mechanism is purely mechanical and topical. The powder is “highly absorptive” and works in three main ways:
Absorption & Concentration: When the powder hits the blood, it rapidly absorbs water. This action concentrates the body’s own platelets, red blood cells, and clotting factors at the bleeding site.
Clot Initiation: This concentration “initiates coagulation” and “accelerates” the natural clotting and fibrin polymerization times.
Mechanical Barrier: The powder forms a stable “mechanical barrier” over the wound, which helps stabilize the new blood clot.
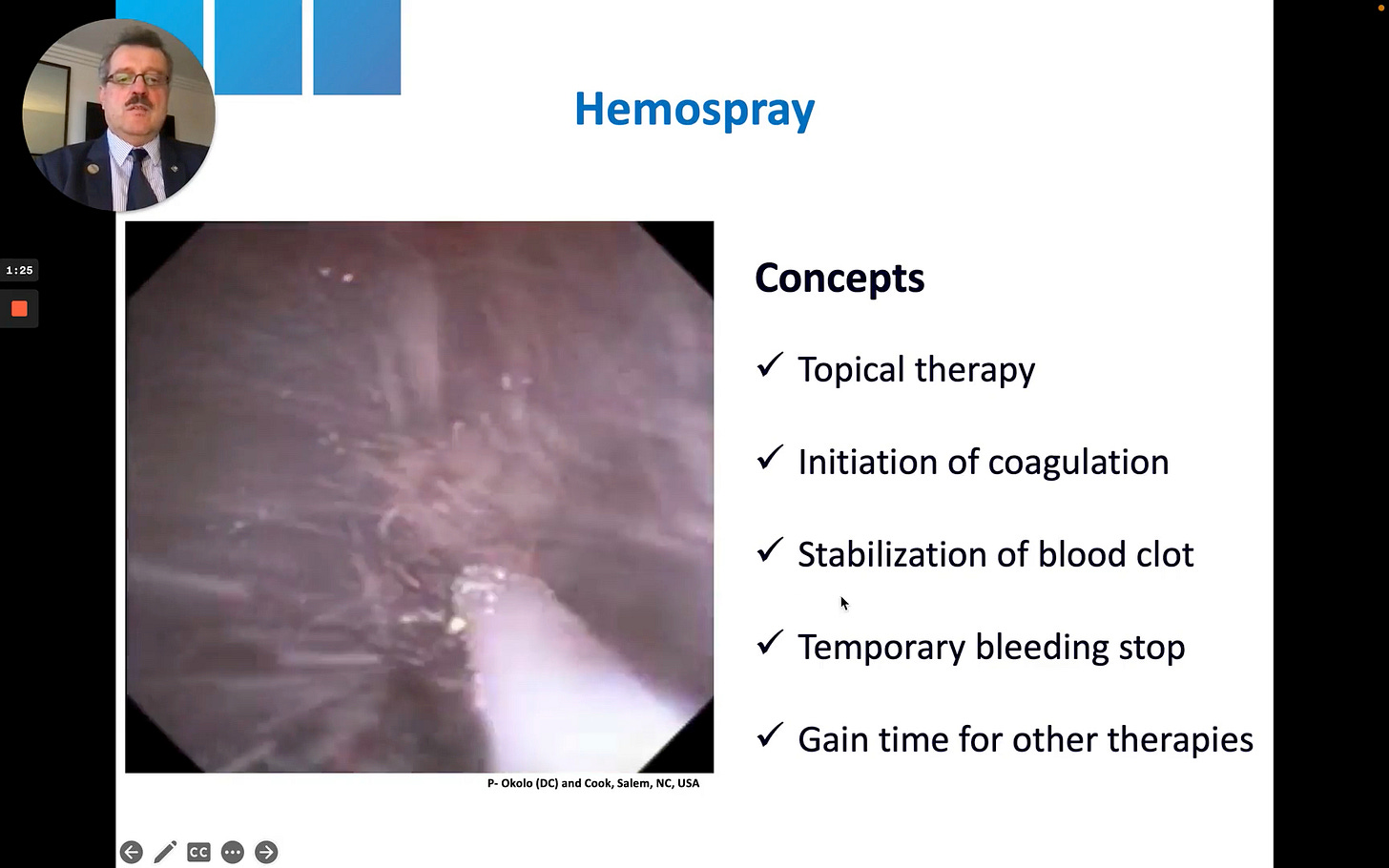
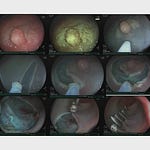
The video shows the white catheter tip near a bleeding vessel. A sudden, forceful burst of white powder (Hemospray) erupts from the catheter, propelled by CO2. The force of the application completely obscures the endoscopic field in a “white out” before the powder settles. This demonstrates the explosive, non-targeted nature of this first-generation application system.
Key Clinical Applications
Professor Mönkemüller highlights two primary use cases for this type of powder.
1. “Down-Staging” an Active Bleed
This is perhaps the most valuable application. In a video from Prof. Jacques Bergman, a patient has a spurting ulcer that continues to bleed despite initial therapy. Hemospray is applied, which achieves temporary hemostasis. This “buys time” and successfully “down-stages” the bleed from an active spirt to a controlled, oozing lesion. This controlled field then allows the endoscopist to successfully apply definitive therapy, such as clips, which may have failed on the actively spurting vessel.
2. Rescue Therapy The powder is also effective as a rescue therapy for iatrogenic bleeding. The speaker shares a case of post-polypectomy bleeding in a patient who was later found to have acquired von Willebrand disease. After conventional therapies failed, Hemospray was applied to stabilize the bleeding site.
Clinical Pearl: Data on Non-Variceal GI Bleeding
The speaker cites a 2022 study (Lau J Y et al., Ann Intern Med) that found Hemospray was “not inferior” to standard endoscopic therapy for non-variceal upper GI bleeding. The study of 224 patients showed no significant difference in recurrent bleeding within 30 days (8.1% for Hemospray vs. 8.8% for control).
A Critical Limitation: Variceal Bleeding
The speaker issues a strong warning against using Hemospray for variceal bleeding.
He references a 2022 pilot study (Ibrahim M et al., Gut) that attempted to use Hemospray for this indication. While it did stop bleeding, the speaker notes the study was “problematic” and the results are not reproducible in most clinical practices for several reasons:
Rapid Endoscopy: The intervention group received endoscopy within 2 hours of presentation, while the control group waited up to 24 hours. This is not a feasible protocol for most hospitals without a dedicated on-call team.
Scope Requirement: The study used a 10 Fr catheter, which requires a therapeutic gastroscope. Many practices do not have these readily available for an emergent bleed.
Flawed Comparison: The control group was not managed according to Baveno guidelines.
“In my eyes,” Prof. Mönkemüller concludes, “Hemospray is not an option for variceal bleeding”.
To see Professor Mönkemüller’s analysis of the 2nd and 3rd-generation powders, including the “powder-gel” concept that offers a much more controlled application, consider becoming a paid subscriber.